Figure 5: Mammary adenocarcinoma in 1-year-old female rat (Petunia).
Case history and photos
History
An approximately 1 year old spayed female PEW named Petunia, adopted from a local humane society, previous history unknown.
Clinical Signs
A small mass was noted in the right axillary region, just behind the right forelimb. The mass was assumed to be a mammary tumor. The mass gradually increased in size over a 4 month period to approximately 2 inches in diameter before the decision was made to proceed with surgery.
Diagnosis
Right axillary mass of unknown etiology attached to skin.
Treatment
Surgical excision of the mass proved somewhat difficult as the mass was not encapsulated and was well attached to the skin, requiring excision of portions of the skin along with the mass. Post op injections of buprenorphine, meloxicam, and Baytril were given. Baytril was prescribed .5ml orally for 14 days following surgery. Meloxicam was prescribed orally for 7 days. Metronidazole pills were prescribed to be crushed and make a paste to use over incision to prevent licking or chewing of incision site. Immediate recovery from surgery was without incident.
Outcome
Following a successful surgery, a return of mass along surgical incision was noted approximately 2 weeks after surgery and the decision was made to again remove the mass. However in the week following the decision the mass grew substantially and surgery was no longer expected to be successful. The owner elected to carefully monitor the mass and its impact on the rat’s quality of life. After a further week with the mass growing at an accelerated rate the determination was made to euthanize, secondary to lack of mobility, lethargy, and diminished appetite.
Follow Up
A biopsy with histopathology of the original mass was obtained:
Histopathology Report
BIOPSY
A biopsy of a right axillary lesion is submitted from a young, mature adult, altered female rat with the gross description of a multi-lobulated, invasive-appearing mass. The biopsy specimen received in formalin at the laboratory approximates 5.0 x 3.5 x 4.0 cm in dimension. Following a necessary interval of additional formalin fixation, the lesion is prepared for histologic processing by complete full-thickness perpendicular cross-sections.
MICROSCOPIC DESCRIPTION
This lesion is a disorganized proliferation of suboptimally differentiated glandular epithelium. The latter is characterized cytomorphologically as basaloid (glandular epithelial reserve cells) generally with a small quantity of basophilic-staining, amorphous cytoplasm surrounding an ovoid to laterally flattened nucleus. Marginated chromatin creates an open-faced/vesicular appearance and exposes nucleoli variable, influenced by the density of cellular compaction versus slight dissociation. There is occasional differentiation of these glandular epithelium reserve cells into larger more lightly eosinophilic-staining secretory glandular epithelial cells. Mitoses are present but relatively infrequent, identified in the average incidence of less than one mitotic spindle per standard quantitated microscopic surface area of 10 consecutive 400 x high-power magnification fields. The glandular epithelium exists in multiple forms, including compactly cellular nests and columns. These occasionally differentiate into rudimentary acini and tubules characterized by reasonably good to poor cellular polarity with considerable cellular crowding and piling.
Tubuloglandular basement membrane circumscription is inconsistent and incomplete. Therefore, there is multifocal disorganized infiltration of the intralesional fibrous connective tissue stroma by the neoplastic epithelium. The lesion contains several micro-and micro-cavitary chambers into which there are disorganized papilliferous proliferations of epithelium. The mass enlarges by both expansion and local infiltration, although the majority of the latter occurs within the interior of the mass rather than around its periphery. Nevertheless, there are a few small satellite nests of neoplastic glandular epithelium surrounding the primary tumor nodule.
Foci of liquefactive necrosis are non-inflammatory. The lesion is restricted to the subcutis. It appears to be confined with biopsy margins, although the peripheral border of normal clean connective tissue surrounding this mass is variable from moderately generous to extremely thin. In some instances the neoplastic glandular epithelium and the biopsy margins appear to coincide. However, there is no evidence of either intra- or peri-lesional vascular infiltration.
MICROSCOPIC FINDINGS
Mammary adenocarcinoma, papillary/cystic with multifocal necrosis and perilesional satellite neoplastic nodules.
COMMENTS/SUMMARY
The axillary region is a common site for mammary glandular epithelium in rodents with potential for neoplastic transformation in the axillary, lateral thoracoabdominal, and flank regions as well as the ventral abdomen. The lesion is sub-classified as a moderate grade malignancy on the basis of cellular undifferentiation, disorganization, necrosis, and invasive growth, although the majority of the latter appears to occur with the intra-lesional supporting fibrous trauma rather than the perilesional subcutis. However, there are a few small satellite nodules around the periphery of the main mass (local perilesional metastases). The thickness of the surgical margin is variable from moderate to minimal with neoplastic glandular epithelium focally extending extremely close to and focally reaching the biopsy margins. Clinical behavior is sometimes more aggressive than might be expected based upon microscopic features such as these. Clinical options might include prophylactic deeper excision or at least very close conservative observation of the surgical site. Lesions of this type have a variable potential for metastasis, the likelihood which is unknown.
Photos
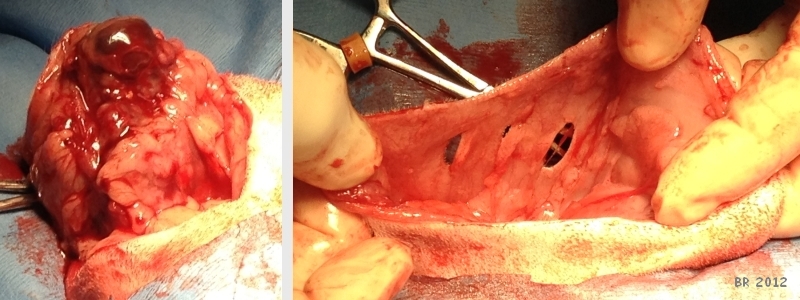 Photo on left shows preparing to remove mass. The photo on the right shows the extent of the area from which mass was removed. |
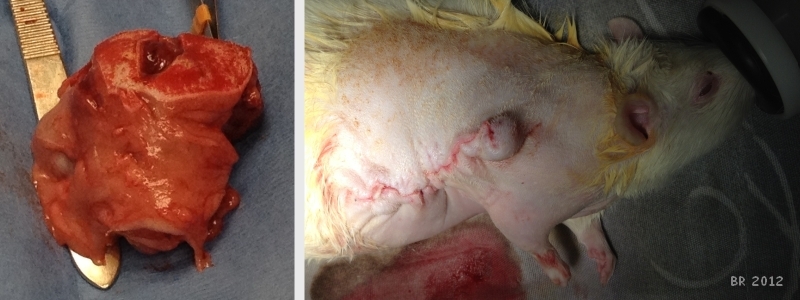 Photo on left shows mass (confirmed adenocarcinoma). The photo on right shows closed incision site and Petunia being recovered. |
Veterinary Surgeon: Kimberly Somjen,DVM
Pathologist: Ken Mero,DVM, PhD
Case history & photos: Branwen Resop


